What a professional spirometry machine must deliver in practice
A professional spirometry machine delivers repeatable, clinically defensible lung function measurements across varied patient conditions. Its role is not to generate curves, but to produce data that can be trusted for diagnosis, monitoring, and comparison over time. Every design decision should support consistency rather than convenience.
Professional spirometry devices perform reliably despite imperfect technique, environmental variation, and operator differences. Systems that only work well under ideal conditions fail real clinical workflows. Clinical accuracy is the outcome of system-wide reliability, not a single component.
Why accuracy defines the value of spirometry equipment
Accuracy determines whether spirometry results can meaningfully guide clinical decisions. Small deviations in measured flow or volume can shift values across diagnostic thresholds. Professional spirometry equipment minimizes both systematic error and random variability.
Accuracy is influenced by multiple factors working together:
- Sensor resolution and responsiveness
- Flow linearity across low and high ranges
- Resistance characteristics during forced maneuvers
- Temperature and humidity compensation
Weakness in any of these areas undermines trust in the entire spirometry system.
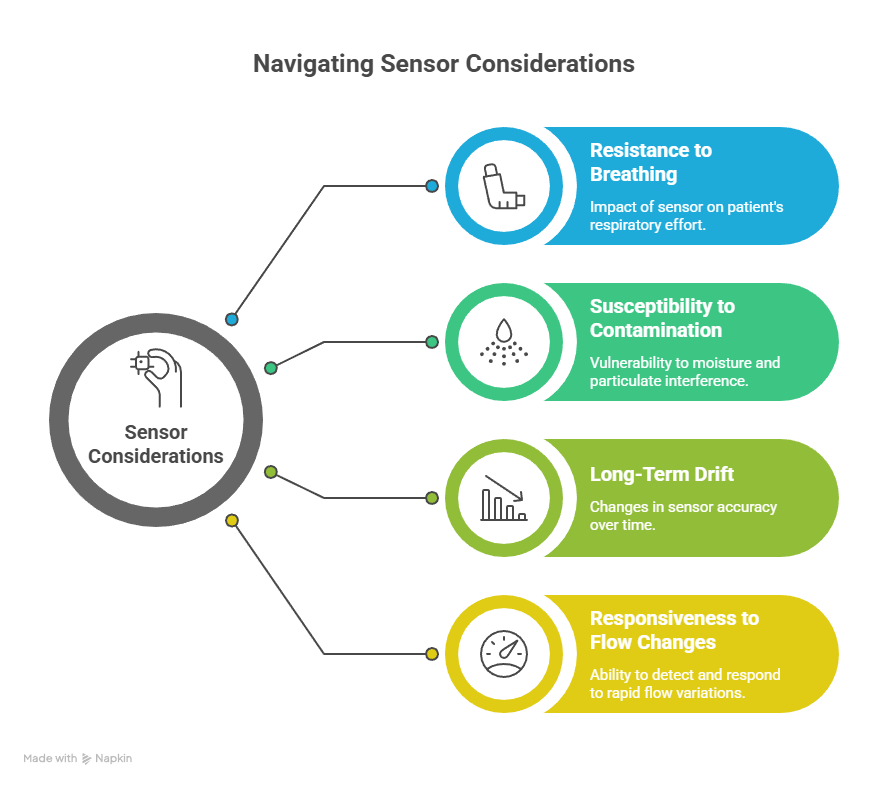
Sensor design and its effect on spirometry device performance
Sensor architecture directly shapes how airflow is captured and interpreted. Professional spirometry devices use sensor technologies chosen for stability, low resistance, and durability under repeated use. The goal is consistent signal fidelity rather than peak sensitivity under narrow conditions.
Common sensor considerations include:
- Resistance added to patient breathing
- Susceptibility to condensation or debris
- Long-term drift under routine cleaning
- Responsiveness during rapid flow changes
Professional systems favor designs that preserve waveform integrity across thousands of tests.
Repeatability as a clinical requirement, not a preference
Repeatability reflects whether a spirometry machine produces the same result under the same conditions. High repeatability enables meaningful trend analysis and disease monitoring. Without it, changes in results may reflect device variability rather than patient physiology.
Professional spirometry equipment maintains repeatability across:
- Different operators
- Multiple test sessions
- Extended periods of use
This stability is essential for longitudinal care and comparative assessment.
Flow and volume range expectations in spirometry systems
A professional spirometry system must accurately measure both very low and very high flow rates. Pediatric patients, elderly individuals, and those with severe obstruction all stress different parts of the measurement range. Narrow operating windows introduce bias.
A clinically capable spirometry machine supports:
- Low-flow detection without signal noise
- High-flow measurement without saturation
- Linear performance across the full range
Devices that perform well only in average adult populations limit clinical usefulness.
Calibration stability and why it matters clinically
Calibration links measured airflow to actual volume movement. Professional spirometry machines are designed to maintain calibration stability over time rather than requiring constant adjustment. Stable calibration reduces workflow friction and error risk.
Key calibration characteristics include:
- Resistance to drift between calibration checks
- Clear indicators of calibration status
- Protection against silent calibration failure
Calibration reliability directly affects confidence in every test result.
Manual versus automated calibration approaches
Manual calibration provides transparency but depends on consistent technique. Automated calibration reduces operator variability but must be internally validated. Professional spirometry systems combine automation with clear verification indicators.
The defining factor is not method choice, but whether calibration status is unmistakable at the point of testing.
Software interpretation as part of spirometry machine accuracy
Spirometry software determines how raw airflow signals become clinical metrics. Professional spirometry machines preserve waveform detail rather than smoothing away diagnostically relevant features. Data processing should enhance clarity, not mask irregularities.
Effective interpretation software:
- Identifies artifacts such as coughs or leaks
- Flags suboptimal maneuvers
- Preserves raw curves for review
Systems that overcorrect or auto-accept poor technique reduce diagnostic reliability.
Workflow integration and operator usability
Usability influences whether spirometry is performed correctly and consistently. Professional spirometry devices are designed for routine clinical workflows, not occasional screening. Efficient operation supports quality without increasing cognitive load.
Workflow-ready systems emphasize:
- Clear test progression
- Minimal setup friction
- Logical navigation during testing
Poor usability encourages shortcuts that degrade data quality.
Interface clarity and real-time feedback
Clear interfaces reduce training burden and operator error. Professional spirometry equipment provides real-time visual feedback during maneuvers to support patient coaching. Feedback should guide, not distract.
Effective interfaces improve test quality while preserving clinical judgment.
Data integrity and longitudinal reliability
Professional spirometry systems preserve raw data alongside calculated values. This ensures that results remain interpretable if standards or clinical questions change. Systems that store only final metrics limit future review.
Data integrity also requires protection against loss or corruption. Reliable spirometry equipment supports continuity across years of patient care.
Connectivity expectations for modern spirometry machines
Clinical environments depend on system integration. Professional spirometry devices support secure data transfer and standardized export formats. Manual transcription introduces risk and inefficiency.
Connectivity enables:
- Seamless record inclusion
- Reduced administrative burden
- Improved care coordination
Professional systems treat connectivity as a core requirement, not an accessory.
Portability versus stability in spirometry equipment design
Portability expands where spirometry can be performed, while stability ensures accuracy. Professional spirometry machines balance both without compromising measurement integrity. Lightweight design should not introduce fragility or drift.
Clinical-grade portable spirometry equipment maintains performance consistency across settings. Stability under movement and transport is a defining quality marker.
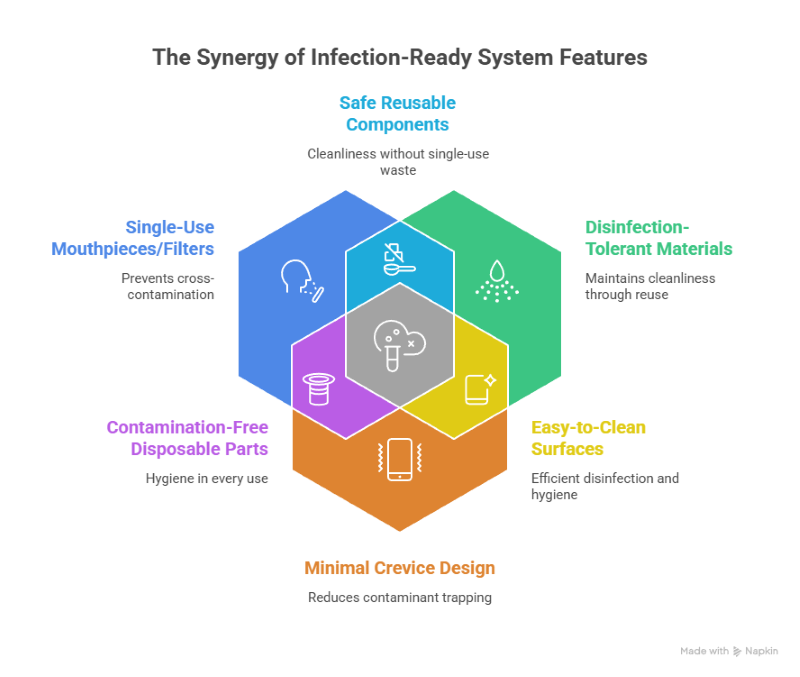
Infection control compatibility in spirometry systems
Spirometry involves forceful exhalation, making infection control critical. Professional spirometry machines support disposable components and repeatable cleaning protocols. Design decisions directly affect cross-contamination risk.
Infection-ready systems provide:
- Single-use mouthpieces or filters
- Materials tolerant of repeated disinfection
- Minimal crevices that trap contaminants
Accuracy must remain intact after cleaning cycles.
Environmental tolerance under real clinical conditions
Temperature, humidity, and airflow vary across clinical spaces. Professional spirometry equipment compensates internally for environmental change. Devices that require controlled conditions increase operational burden.
Environmental resilience reduces false variability and supports consistent testing outcomes. Professional systems absorb environmental noise rather than amplifying it.
Pediatric and special population performance
Professional spirometry machines must accommodate patients with limited cooperation or atypical breathing patterns. Sensitive low-flow detection and fast response times are essential. Adult-focused devices often fail in pediatric or compromised populations.
Clinical readiness includes inclusive performance. Professional spirometry systems anticipate variability rather than assuming ideal execution.
Durability and expected lifecycle of spirometry equipment
Professional spirometry equipment is expected to operate reliably for years. Durability affects both cost and clinical trust. Frequent failures disrupt care and erode confidence in results.
Lifecycle durability includes:
- Mechanical robustness
- Sensor longevity
- Ongoing software stability
Short-lived devices rarely meet professional expectations.
Maintenance burden and operational reality
Maintenance requirements influence whether spirometry is used consistently. Professional spirometry machines minimize routine upkeep while making issues visible. Predictable maintenance supports compliance.
Excessive servicing discourages proper use. Professional systems balance simplicity with accountability.
Built-in quality controls and validation checks
Professional spirometry systems incorporate internal checks that validate performance during use. These controls detect anomalies before results are finalized. Quality safeguards protect against unnoticed device failure.
Quality validation reinforces confidence for both operators and clinicians. Trustworthy systems confirm their own readiness.
Reporting flexibility without data distortion
Clinical reporting needs vary by specialty. Professional spirometry machines offer reporting flexibility without altering measured values. Presentation should clarify, not reinterpret, results.
Effective reports remain faithful to underlying data while supporting clinical communication.
Training implications of spirometry system design
Professional spirometry systems are designed to be learned efficiently without oversimplification. Clear workflows reduce training time while maintaining rigor. Overly complex systems increase inconsistency.
Design-driven clarity supports standardized testing across staff members.
Regulatory alignment as a baseline expectation
Professional spirometry machines align with recognized clinical and regulatory expectations. Compliance ensures defensible results within healthcare systems. Falling short introduces downstream risk.
Regulatory alignment is a minimum requirement, not a differentiator.
Scalability across practice sizes and settings
Professional spirometry systems adapt to different clinical volumes without redesign. Scalability protects long-term investment and avoids premature replacement. Flexible systems grow with practice needs.
Scalability reflects thoughtful system architecture rather than feature overload.
Evaluating total cost beyond the purchase price
Upfront cost rarely reflects long-term value. Professional spirometry machines are evaluated on total ownership cost, including maintenance, consumables, and lifespan. Low purchase prices often conceal future expense.
Sustained performance defines value more than initial savings.
Cost comparison considerations
| Cost Factor | Professional Systems | Basic Devices |
| Measurement stability | High | Variable |
| Maintenance frequency | Predictable | Often higher |
| Lifecycle length | Long-term | Shorter |
| Clinical reliability | Consistent | Inconsistent |
Decision criteria that separate professional spirometry systems
Professional spirometry systems distinguish themselves through resilience and consistency. Basic devices may capture airflow but lack clinical defensibility. The difference lies in performance under non-ideal conditions.
Key differentiators include:
- Repeatable accuracy
- Workflow compatibility
- Data integrity
- Long-term reliability
Professional systems support clinical judgment rather than forcing adaptation.
Common misconceptions when selecting spirometry equipment
A common misconception is that all spirometry devices measure the same thing equally well. In practice, design choices create meaningful performance differences. Professional systems address variability directly.
Another misconception is that portability implies lower quality. Some professional spirometry machines combine portability with clinical-grade accuracy.
Evaluating spirometry devices during demonstrations
Hands-on evaluation reveals qualities that specifications cannot. Professional spirometry machines show stable curves, responsive feedback, and intuitive operation. Inconsistencies become apparent quickly.
Demonstrations should focus on repeatability, calibration behavior, and artifact handling rather than feature lists.
Long-term support as part of spirometry system value
Professional spirometry equipment depends on sustained software and service support. Long-term availability protects data continuity and workflow stability. Unsupported systems become operational risks.
Support quality influences uptime, confidence, and adoption. Professional systems are designed with continuity in mind.
Why BOMImed Is an Optimal Supplier for Professional Spirometry Systems
BOMImed is an optimal supplier because its spirometry offerings are aligned with clinical accuracy, workflow reliability, and long-term equipment performance. The spirometry machines and systems available through BOMImed are selected for professional environments where repeatability, durability, and defensible data matter more than feature volume.
BOMImed focuses on spirometry devices that integrate cleanly into real clinical workflows. This includes systems that balance portability with measurement stability, support consistent calibration behavior, and preserve data integrity over time. The result is spirometry equipment that performs reliably under routine, high-use conditions rather than only in controlled settings.
Supplier value also comes from operational consistency. BOMImed offers a cohesive spirometry systems portfolio that scales across practice sizes while maintaining standardized accessories, disposables, and support expectations. That consistency reduces procurement friction and simplifies long-term equipment management.
For clinics prioritizing dependable spirometry data, sustainable workflows, and professional-grade reliability, BOMImed represents a supplier choice built around clinical performance rather than short-term convenience.
People Also Ask About Spirometry Machines
What is a spirometry machine used for?
A spirometry machine measures airflow and lung volume during controlled breathing maneuvers to assess respiratory function and detect abnormal patterns.
How accurate are professional spirometry devices?
Professional spirometry devices are designed for consistent, repeatable measurements when properly calibrated and operated within clinical workflows.
What is the difference between spirometry equipment and spirometry systems?
Spirometry equipment refers to the physical device, while spirometry systems include hardware, software, and data management working together.
Are portable spirometry machines clinically reliable?
Portable spirometry machines can be clinically reliable when designed for professional use and built to maintain calibration stability.
How often does spirometry equipment need calibration?
Calibration frequency depends on device design and usage, but professional spirometry machines maintain stability and clearly indicate when recalibration is required.
Can one spirometry device be used for adults and children?
Many professional spirometry devices support both adult and pediatric testing through wide flow-range sensitivity.
What matters most when choosing spirometry equipment for a clinic?
Accuracy, repeatability, workflow fit, data integrity, and durability matter more than feature count or appearance.