Spirometry devices are the backbone of modern respiratory diagnostics. They measure how much air a person can inhale and exhale, and how quickly it moves through the lungs — data essential for diagnosing and managing conditions like asthma, COPD, and occupational lung diseases. But beyond clinical diagnostics, spirometers have become a strategic investment for hospitals, occupational health programs, and telehealth providers looking to standardize quality and efficiency in respiratory testing.
These devices are no longer confined to specialized labs. With the rise of portable, wireless, and calibration-free models, spirometry has moved closer to the point of care — even reaching patients at home. Understanding how these devices differ, what standards they must meet, and how to integrate them effectively can save organizations time, money, and liability.
What a Spirometry Device Actually Measures
Summary: Spirometry measures airflow and lung volumes, translating patient effort into numerical and graphical data that reveal how well the lungs are functioning.
When a patient exhales forcefully into a spirometer, the device records key data points:
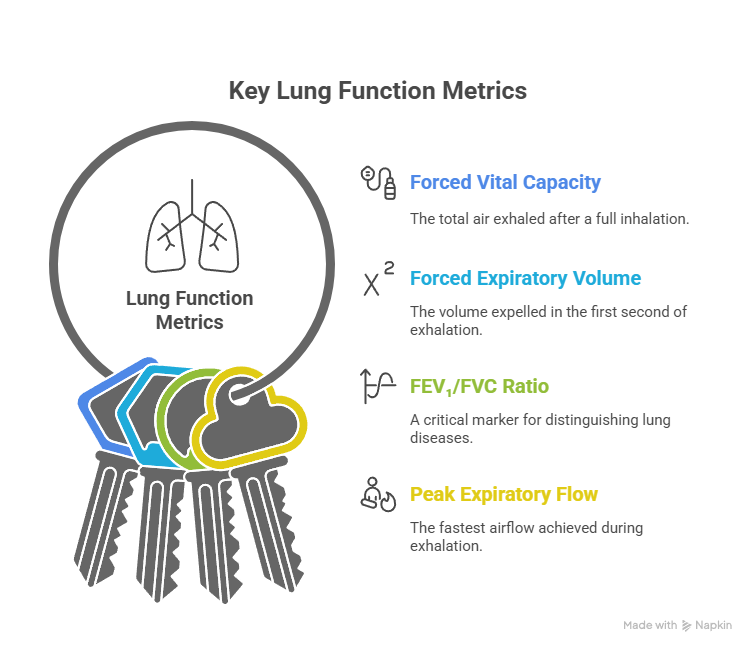
- FVC (Forced Vital Capacity): The total air exhaled after a full inhalation.
- FEV₁ (Forced Expiratory Volume in One Second): The volume expelled in the first second of exhalation.
- FEV₁/FVC Ratio: A critical marker for distinguishing obstructive from restrictive lung diseases.
- PEF (Peak Expiratory Flow): The fastest airflow achieved during exhalation.
Modern spirometers display these results as both flow-volume and volume-time curves. By analyzing the shape and symmetry of these curves, clinicians can detect abnormalities, assess disease severity, and evaluate treatment response.
For administrators and clinical leaders, understanding these metrics isn’t just academic — it’s a compliance issue. Reimbursement codes, quality audits, and even occupational health regulations depend on precise, reproducible data.
The Three Core Types of Spirometry Devices
Summary: Every spirometer measures airflow, but the underlying technology determines accuracy, maintenance needs, and total cost of ownership.
1. Pneumotachograph (Differential Pressure)
Pneumotach-based devices measure airflow by detecting the pressure difference across a fixed resistance. Known for their accuracy and stability, they are often used in hospitals and pulmonary function labs. They require routine cleaning and daily verification but excel in environments that demand repeatable precision.
2. Turbine-Based Spirometers
These devices use a rotating vane or turbine that spins as air passes through, translating mechanical motion into flow readings. They’re durable, portable, and relatively low-maintenance — ideal for primary care offices or field screenings. However, moving parts can accumulate residue over time, requiring occasional recalibration.
3. Ultrasonic Spirometers
Ultrasonic models measure airflow using high-frequency sound waves. They have no moving parts, minimal drift, and are often marketed as “calibration-free.” While typically more expensive, their long-term reliability and reduced maintenance make them popular for multi-site health systems and occupational testing networks.
Categories of Spirometry Devices by Use Case
Summary: Choosing a spirometry device depends on your clinical workflow — from hospital-grade diagnostics to home rehabilitation programs.
Clinical Diagnostic Spirometers
- Designed for full pulmonary function testing in hospitals and specialty clinics.
- Offer complete parameter sets, flow-volume loops, and bronchodilator response testing.
- Often include EMR connectivity, automatic grading, and advanced quality feedback.
Portable or Handheld Spirometers
- Lightweight, battery-powered, and designed for point-of-care or outreach use.
- Perfect for occupational health programs, mobile clinics, or primary care settings.
- Many now feature Bluetooth connectivity and cloud reporting.
Incentive Spirometers
- Simple, mechanical devices that encourage slow, deep breathing — used primarily after surgery or illness.
- Not diagnostic but crucial for preventing lung complications like atelectasis.
- Often issued to patients for home use or post-operative recovery.
How Spirometry Devices Work in Practice
Summary: The workflow behind a valid spirometry test combines patient coaching, device calibration, and strict adherence to standards.
A typical test includes:
- Calibration Verification: The operator confirms device accuracy using a 3-liter calibration syringe.
- Patient Preparation: The patient is seated upright, nose clipped, and instructed on correct breathing technique.
- Test Execution: The patient performs at least three valid forced expiratory maneuvers.
- Quality Grading: The system assigns an A–F grade based on effort, repeatability, and error detection.
- Result Interpretation: The clinician evaluates FEV₁, FVC, and flow-volume curves against predicted norms.
Well-designed spirometers guide the operator through this workflow, flagging errors such as early termination, coughing, or air leaks. For managers, devices with built-in quality feedback reduce the need for repeat tests and minimize data variability across technicians.
How to Choose the Right Spirometry Device for Your Facility
Selecting the best spirometer isn’t about finding the newest or most expensive model—it’s about matching the device to your testing volume, accuracy needs, and workflow integration goals.
For most healthcare organizations, spirometry purchasing begins with price comparisons and ends with buyer’s remorse. The market is crowded with devices that promise compliance, connectivity, and “no calibration needed” convenience. The truth is that no single spirometer fits every environment. Hospitals, urgent care centers, and occupational clinics each require different configurations. The key is understanding how and where the device will be used before evaluating features.
Step 1: Define Your Primary Use Case
Before comparing models, clearly outline what the spirometer will support:
- Diagnostic testing for pulmonology or pre-surgical evaluation.
- Occupational screening for workers in industrial or environmental roles.
- Rehabilitation or telehealth programs that monitor chronic respiratory conditions remotely.
Each use case comes with distinct accuracy requirements, data-handling needs, and regulatory obligations. For example, a hospital lab performing pre-operative testing must meet full ATS/ERS calibration and reporting standards. A mobile wellness clinic may prioritize portability and battery life over advanced analytics.
Step 2: Evaluate Accuracy and Standards Compliance
The most critical feature in any spirometry device is adherence to established performance standards. Models that conform to ISO and ATS/ERS guidelines ensure accuracy within ±2.5% and reproducibility across operators. Look for devices that:
- Offer automatic quality grading to flag invalid efforts.
- Include 3-liter calibration verification compatibility for daily checks.
- Display real-time flow-volume loops for operator feedback.
If a vendor cannot clearly demonstrate compliance with international standards, the device should not be considered for medical diagnostics. Compliance determines not just accuracy, but insurability and defensibility of test results.
Step 3: Consider Portability and Workflow
Portability affects workflow efficiency as much as accuracy does.
- Fixed desktop systems work best in labs and high-volume clinics where stable calibration and wired connectivity matter most.
- Portable or hybrid systems provide flexibility for outreach, home visits, and multi-department use.
- Handheld Bluetooth spirometers are best suited for telehealth programs and occupational screenings where rapid setup and cloud reporting are valuable.
Each form factor has operational trade-offs. Portable systems often sacrifice print capabilities, while desktop units may require dedicated operator space and power sources.
Step 4: Review Data Integration and Connectivity
Spirometry data is most valuable when seamlessly integrated into clinical systems.
Prioritize devices that:
- Export in standardized formats (PDF, XML, HL7).
- Integrate directly with major EMRs or occupational health platforms.
- Support wireless data transfer or cloud synchronization for multi-site monitoring.
Even a 10-second delay per upload can translate into hours of lost productivity across hundreds of tests per week. Modern spirometers that automate record saving and reporting minimize administrative friction and improve compliance documentation.
Step 5: Factor in Maintenance, Consumables, and Training
Budgeting for spirometry extends beyond the sticker price. Maintenance and consumable costs can significantly impact long-term ROI.
- Disposable filters, mouthpieces, and syringes should be readily available and affordable in bulk.
- Devices with non-washable flow sensors should include service contracts or replacement programs.
- Choose vendors that provide technician training, certification modules, or remote support—especially if spirometry testing is new to your facility.
Step 6: Project Total Cost of Ownership
The lowest purchase price often hides the highest maintenance burden. A $900 spirometer may cost more than a $2,500 model over five years once filters, calibration kits, and downtime are accounted for.
Estimate annual consumable costs, staff time, and expected lifespan before finalizing your choice.
Step 7: Align with Future Standards
Spirometry is evolving rapidly with race-neutral reference equations, telemonitoring capabilities, and AI-assisted quality control.
Selecting a model that supports software updates and firmware upgrades ensures compliance when new guidelines emerge. This future-proofs your investment and reduces the risk of obsolescence.
How Spirometry Devices Are Used Across Healthcare and Workplace Settings
Spirometry is no longer limited to pulmonary labs. Hospitals, primary care networks, and employers use these devices to improve patient outcomes, maintain compliance, and protect workforce health.
Hospitals and Specialty Clinics
In hospital environments, spirometry devices serve as front-line diagnostic tools. Pulmonologists and anesthesiologists use them to:
- Diagnose asthma, COPD, and restrictive lung diseases.
- Evaluate lung function before surgeries involving anesthesia or chest procedures.
- Monitor patients recovering from pneumonia, COVID-19, or respiratory distress.
Hospitals often deploy multiple spirometry systems networked into a single data environment. Integration with patient records allows clinicians to view trends over time, supporting early intervention and outcome tracking. High-end desktop spirometers, often pneumotach-based, provide the precision needed for comprehensive pulmonary function testing and are backed by continuous calibration verification.
Primary and Community Care
In primary care, spirometry devices enable early detection of chronic lung conditions before symptoms progress. Clinicians can quickly determine if breathing issues stem from asthma, smoking-related COPD, or other causes.
Modern portable devices have made it possible to conduct these tests in routine appointments, with results instantly available for interpretation. When coupled with cloud reporting, general practitioners can share results with specialists without the delays of manual documentation.
Urgent Care and Occupational Health
Occupational medicine relies heavily on spirometry to assess worker fitness, detect early signs of occupational lung disease, and document compliance with safety standards.
Portable or hybrid spirometers are particularly valuable for:
- Construction and manufacturing workforces exposed to dust or fumes.
- Transportation and logistics companies requiring respiratory clearances.
- Mining, oil, and chemical industries with mandated surveillance programs.
Because field environments are unpredictable, these devices must withstand travel, variable conditions, and frequent disinfection. Battery life, rugged design, and simple calibration checks are major priorities in these settings.
Telehealth and Remote Monitoring
Telehealth programs increasingly deploy Bluetooth spirometers to monitor chronic respiratory conditions from home.
Patients perform tests under virtual supervision, with results automatically transmitted to care teams. This model reduces unnecessary office visits while maintaining data continuity.
Remote spirometry also enhances clinical trials, enabling decentralized data collection from participants without the constraints of in-person sessions.
Post-Surgical and Rehabilitation Programs
Incentive spirometers, though simple, are essential to recovery programs. They encourage deep breathing, prevent lung collapse, and improve oxygenation following surgery.
Hospitals often issue these devices to patients during discharge, integrating their use into broader rehabilitation protocols. Despite their low cost, adherence remains a challenge—so many programs now include digital reminders or gamified tracking to boost engagement.
Public Health and Screening Programs
Government agencies and nonprofits use spirometry to evaluate lung function in populations exposed to air pollution, wildfires, or workplace hazards.
Field teams use compact spirometers that connect to tablets for on-site data capture. Results help identify communities at risk and inform preventive health initiatives.
Why Multi-Setting Adaptability Matters
Healthcare networks increasingly require spirometry systems that operate seamlessly across multiple settings — from inpatient diagnostics to mobile testing units.
Choosing a device ecosystem that can adapt to both stationary and portable configurations ensures consistent data and reduces training complexity across departments.
Operational Benefits Beyond the Clinic
Spirometry data carries strategic value. Aggregated results reveal trends in population health, environmental exposure, and chronic disease management. For large organizations, this transforms respiratory testing from a regulatory obligation into a data-driven performance indicator.
BOMImed Spirometry Devices: Precision, Performance, and Practicality for Clinical Environments
BOMImed’s spirometry systems combine hospital-grade accuracy with everyday usability—offering reliable lung function diagnostics that align with clinical workflows, compliance standards, and long-term operational value.
BOMImed has become a trusted distributor of advanced medical devices across Canada, and its spirometry systems reflect the same principles that guide its broader product portfolio: precision engineering, intuitive operation, and dependable service support. Designed for hospitals, clinics, and physician practices, BOMImed’s spirometry lineup helps healthcare providers perform fast, accurate, and reproducible lung function tests while maintaining strict adherence to international standards.
Purpose-Built for Hospital and Physician Use
Each system in BOMImed’s spirometry category is built with the clinical environment in mind—prioritizing consistency, hygiene, and data management. These devices are ideal for practices where reliability and throughput matter just as much as measurement accuracy. Whether a pulmonary lab performs dozens of tests daily or a family physician conducts occasional screenings, BOMImed’s systems offer a scalable solution that adapts to varying volumes and patient types.
Core Features Across the Portfolio
BOMImed’s spirometry devices are distinguished by a few essential characteristics that define their performance and usability:
- Clinical Accuracy: Each unit delivers precise, reproducible readings that meet the latest ATS/ERS standards for spirometry.
- Ease of Use: Intuitive interfaces reduce operator error and accelerate workflow for both experienced technicians and first-time users.
- Connectivity: Integration options for EMR systems and digital health platforms make it easy to store, share, and analyze patient data securely.
- Compact, Ergonomic Design: Many devices feature portable configurations suited for multi-room use or bedside diagnostics without sacrificing accuracy.
- Infection Control: BOMImed supplies compatible filters and mouthpieces to maintain strict hygiene compliance in hospital environments.
Trusted Technologies from Leading Manufacturers
BOMImed partners with world-class manufacturers known for their engineering and innovation in respiratory diagnostics. Their spirometry systems typically incorporate:
- Ultrasonic Flow Sensors that eliminate moving parts and reduce maintenance.
- Turbine-Based Measurement Systems offering high reliability and low operational costs.
- Real-Time Flow-Volume and Volume-Time Displays for visual assessment and quality grading during testing.
- Integrated Calibration Verification Tools to ensure ongoing accuracy across multiple test sessions.
This combination of technologies ensures healthcare providers can deliver precise pulmonary assessments while minimizing downtime and consumable usage.
Solutions Tailored to Hospital Workflows
For hospitals, BOMImed offers spirometry units that seamlessly integrate into broader pulmonary function testing networks. These systems include advanced software for test tracking, automatic report generation, and trend analysis—critical features for respiratory departments managing chronic disease follow-up and pre-surgical assessments.
Physicians benefit from a streamlined setup that fits comfortably within the constraints of outpatient practice. The plug-and-play nature of these devices enables quick deployment, while on-screen prompts guide operators through proper test execution and result validation.
Operational Advantages for Healthcare Teams
Beyond the technology itself, BOMImed’s spirometry solutions emphasize service and sustainability:
- Minimal Maintenance Requirements: Devices are designed to remain calibration-stable over long periods, reducing technician workload.
- Scalable Configurations: Clinics can start with a single device and expand testing capacity as patient demand grows.
- Ongoing Support: BOMImed’s dedicated customer service and technical assistance ensure consistent performance long after purchase.
- Cost Efficiency: With accessible consumables and dependable durability, these systems lower total cost of ownership while maintaining diagnostic integrity.
Designed for Modern Respiratory Programs
From pulmonary specialists to primary care teams, BOMImed spirometry devices serve the evolving needs of modern respiratory medicine. They enable hospitals and clinics to:
- Perform baseline assessments for preoperative patients or chronic disease management.
- Conduct bronchodilator response testing with repeatable, high-quality measurements.
- Implement standardized screening programs for occupational or community health initiatives.
- Support longitudinal data tracking for patient monitoring and research.
The result is a spirometry platform that is not only clinically sound but operationally sustainable—one that aligns with the growing demand for efficiency, digital integration, and patient-centered care.
Global Standards and Compliance Requirements
Summary: Compliance with ATS/ERS and ISO standards ensures data consistency across patients, sites, and devices.
- ATS/ERS Standards (2019): Define testing procedures, calibration, acceptability criteria, and quality grading.
- ISO 26782: Establishes mechanical and accuracy requirements for devices measuring respiratory flow in humans.
- OSHA/NIOSH Guidelines: Govern spirometry use in occupational surveillance and industrial hygiene programs.
For hospitals, compliance isn’t optional — it’s tied to accreditation and reimbursement. For employers or wellness providers, adherence protects against liability when evaluating worker respiratory health.
Key Features to Look For in Spirometry Devices
Summary: The most accurate spirometer isn’t always the most expensive — it’s the one aligned with your testing goals, data systems, and workflow.
Accuracy and Standards
- Must meet ATS/ERS 2019 standards and ±2.5% accuracy.
- Should include automated quality grading and calibration reminders.
Ease of Use
- Intuitive interfaces with visual feedback for patients and operators.
- Real-time curve display for immediate quality assessment.
Connectivity
- Integration with EMR or occupational health software via USB, Bluetooth, or HL7 interface.
- Secure cloud data storage for remote monitoring or tele-spirometry.
Maintenance and Consumables
- Availability of disposable filters, mouthpieces, and B/V filters for infection control.
- Clear documentation for cleaning and servicing to maintain compliance.
Portability and Flexibility
- For outreach programs or telehealth, lightweight designs with rechargeable batteries are essential.
- Hybrid models that function both as standalone and PC-connected devices maximize ROI.
Operational and Financial Considerations
Summary: A spirometry program’s success depends as much on implementation and cost structure as on the device itself.
Initial Investment
Device prices range widely:
- Incentive spirometers: under $20.
- Portable handheld models: $500–$1,500.
- Full diagnostic systems: $2,000–$4,000+.
Consumables
Budget for disposable filters, mouthpieces, and calibration syringes — recurring costs that can exceed the device price over its lifetime.
Maintenance
Annual calibration verification or manufacturer servicing keeps results defensible in audits. Selecting models with low maintenance requirements can offset upfront costs.
Integration Costs
If your EMR or occupational health platform doesn’t directly interface with the spirometer, data entry time adds hidden costs. Investing in compatible systems often pays off in labor savings.
Implementation Workflow and Quality Control
Summary: Establishing a structured workflow ensures reliable data, fewer repeats, and consistent patient experiences.
- Staff Training
Train all operators in proper technique, test coaching, and device handling. Consistent execution is more important than the model itself. - Daily Verification
Check the spirometer with a certified 3-liter syringe at various flow rates to confirm accuracy. - Quality Monitoring
Review test logs regularly to track repeat rates, calibration failures, and quality grades. These metrics form the backbone of quality assurance programs. - Infection Control Protocols
Implement single-use filters, sterilization schedules, and documentation to meet compliance and protect staff and patients. - Reporting and Integration
Ensure test data flows seamlessly into EMRs or reporting dashboards. For occupational health, automated reporting simplifies compliance documentation.
Common Challenges and How to Solve Them
Summary: Even the best spirometer can fail operationally if processes aren’t aligned.
Problem: Inconsistent calibration
Solution: Automate verification reminders, assign accountability to trained staff, and document every check.
Problem: Poor test quality due to patient effort
Solution: Use visual coaching aids and verbal instruction to ensure maximum effort; verify results via flow-volume loops.
Problem: Integration issues with EMR
Solution: Choose devices that support HL7 or direct export. Test data transfer workflows before full deployment.
Problem: High consumable costs
Solution: Negotiate supply contracts or bulk purchasing; standardize filter types across sites to reduce waste.
Frequently Asked Questions: Spirometry Devices
What is the most accurate type of spirometer?
Ultrasonic spirometers generally offer the highest accuracy and lowest drift, though pneumotach models remain the gold standard for lab-based testing.
Can patients use spirometry devices at home?
Yes. Portable, app-connected spirometers are increasingly used for telehealth and chronic care management, provided patients receive proper coaching.
How often should spirometers be calibrated?
Most organizations perform daily verification and periodic full calibration checks as per ATS/ERS standards.
What’s the difference between an incentive spirometer and a diagnostic spirometer?
An incentive spirometer promotes lung exercise after surgery; a diagnostic spirometer measures airflow and volume for clinical evaluation.
Are disposable filters mandatory?
Yes. Single-use bacterial/viral filters are standard infection-control practice in all shared devices.
Advanced Spirometry Devices
Selecting the right spirometry device plays a critical role in ensuring accurate lung function testing and effective respiratory care. Whether for hospital use, private practice, or home monitoring, each spirometer machine offers unique features tailored to different clinical needs. Below is a comprehensive overview of available spirometry products and accessories, including their key functions and ideal use cases, to help you choose the best solution for your testing environment.
| Product Name | Description |
|---|---|
| FlowMIR | A disposable turbine flowmeter designed for hygienic, single-patient use. Ensures accurate readings and reduces contamination risk. |
| Minispir | A compact, USB-connected spirometer that streams real-time data to a PC for detailed analysis. |
| Smart One | A handheld, Bluetooth-enabled device compatible with smartphones for home lung function monitoring. |
| Spirobank II Basic | A cost-effective, stand-alone spirometer with on-screen curves and essential measurements. |
| Spirobank II Smart | An advanced version with Bluetooth connectivity and incentive animations for patient engagement. |
| Spirodoc | A multifunctional touchscreen spirometer with optional oximetry, sleep testing, and continuous monitoring. |
| Spirolab | A full spirometry workstation with touchscreen and built-in printer for high-throughput testing. |
| Cable USB | A standard USB cable for stable and secure PC connectivity with MIR spirometry devices. |
| Calibration Syringe | A precision syringe used to calibrate spirometers to maintain accuracy and compliance. |
| Nose Clips | Comfortable, single-use clips that prevent nasal air leakage during testing. |
Choosing the right spirometry solution depends on your workflow, patient volume, and diagnostic goals. Whether you need a compact handheld device for mobile testing or a full workstation for high-throughput clinical use, BOMImed offers a complete range of spirometry systems and accessories to support accurate, efficient respiratory care. To learn more, request a quote, or speak with a product specialist, contact BOMImed.